Promoted peroxymonosulfate activation by electron transport channel construction for rapid Cu(ii)/Cu(i) redox couple circulation†
Abstract
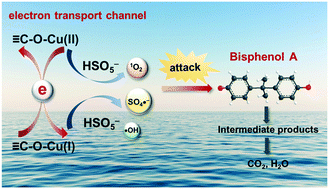
The effective construction of electron transport channels has essential significance for electron-transfer rate enhancement in REDOX processes during peroxymonosulfate (PMS) activation. This study focuses on the establishment of Cu-involved covalent bonds due to the unique electron configuration of Cu ions. The strategy was achieved by anchoring Cu atoms to an O-doped g-C3N4 (Cu–O–CN) matrix. The C–O–Cu linkage was successfully constructed, derived from the precise incorporation of oxygen. From XAFS, H2-TPR, and solid-ESR characteristic observations, the desired REDOX performance was verified. The constructed C–O–Cu linkages were treated as electron transport channels for electron transfer while reacting with PMS, effectively accelerating the REDOX circulation of the Cu(II)/Cu(I) redox couple and promoting the consecutive generation of active species. Consequently, nearly 100% bisphenol A (BPA) was degraded in the Cu–O–CN/PMS reaction system within 30 min. This demonstrates the effectiveness of Cu–O–CN for PMS activation. The prepared Cu–O–CN exhibited definite stability and potential reusability. This research not only highlights the tailored design of heterogeneous catalysts for PMS activation but also broadens its application in water purification.


 Please wait while we load your content...
Please wait while we load your content...