Sustainable photoinduced decarboxylative chlorination mediated by halogen atom transfer†
Abstract
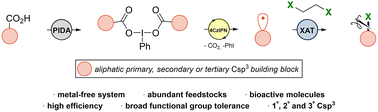
Chlorinated organic backbones constitute important components in existing biologically active chemicals, and they are extraordinary useful intermediates in organic synthesis. Herein, an operationally simple and sustainable halodecarboxylation protocol via halogen-atom transfer (XAT) as a key step is presented. The method merges a metal-free photoredox system with (diacetoxyiodo)benzene (PIDA) as a hypervalent iodine reagent using 1,2-dihaloethanes as halogen sources to afford haloalkanes in an efficient manner. The sustainability of this protocol is highlighted by an important waste recovery protocol as well as by atom economy and carbon efficiency parameters.


 Please wait while we load your content...
Please wait while we load your content...
