Catalysts confined inside CNTs derived from 2D metal–organic frameworks for electrolysis†
Abstract
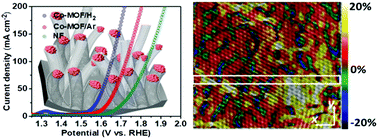
Two-dimensional metal–organic framework (MOF) nanosheets have attracted considerable research interest as electrocatalysts, and thermal annealing is important to boost their conductivity. The effect of annealing atmosphere on the electrochemical performance of 2D MOFs and their catalytic center structure have been investigated. The Co–MOF/H2 synthesized by annealing of 2D MOF under a H2 atmosphere has shown a significantly enhanced catalytic activity compared with those annealed under an Ar atmosphere (Co–MOF/Ar). The Co–MOF/H2 has 2–3 graphitic layers of graphitic carbon coating and presents a large amount of high valent Co2+. H2 annealing leads to a fast reduction of Co–MOF to Co/CoOx nanoparticles and catalyzes the growth of CNTs with MOF feed as carbon source. The Co–MOF/H2 shows a high electrocatalytic activity which requires an overpotential of 312 mV to reach a current density of 10 mA cm−2. A Co–MOF/H2-based water electrolyzer requires a potential of 1.619 V to reach a current density of 10 mA cm−2 for overall water splitting in 1.0 M KOH. After 25 h of continuous operation for water electrolysis, the Co–MOF/H2-based cell has shown a negligible increase in the overpotential, indicating its superior durability compared to the 2D Co–MOF.


 Please wait while we load your content...
Please wait while we load your content...