Methoxy substituents activated carbazole-based boron dimesityl TADF emitters†
Abstract
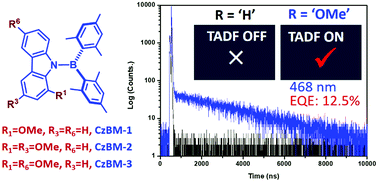
N-Borylated emitters bearing both boron dimesityl acceptor (Mes)2B and phenoxazine or acridine donors are a class of efficient TADF emitters; however, switching to a carbazole donor nullifies the TADF characteristics. This work is targeted at improving the TADF characteristics of the carbazole based (Mes)2B emitters by introducing up to two methoxy substituents at the carbazoles. We hereby report the design and synthesis of three methoxy substituted carbazoles (Cz-1, Cz-2 and Cz-3) and the corresponding directly N-borylated emitters, CzBM-1, CzBM-2 and CzBM-3. Moreover, a p-phenylene spacer was also introduced between the (Mes)2B unit and carbazole, giving CzPBM-2 and CzPBM-3. As confirmed by the bi-exponential transient decay analyses, all title compounds exhibit prominent TADF character, while the parent molecules CzBM-0 and CzPBM-0 without methoxy groups show no TADF characteristics. The results manifest the importance of the donor strength of substituents and degrees of spatial orthogonality in harnessing the charge transfer properties, minimalizing the S1–T1 energy gap and facilitating the reverse intersystem crossing process. OLED devices with doped CzBM-2 and CzPBM-3 emitters exhibited a maximum external quantum efficiency of 12.5% and 13.3%, respectively, confirming the potential of methoxy substituents in improving their TADF characteristics.
- This article is part of the themed collection: Journal of Materials Chemistry C Lunar New Year collection 2021


 Please wait while we load your content...
Please wait while we load your content...