Ultrafine oxygen-defective iridium oxide nanoclusters for efficient and durable water oxidation at high current densities in acidic media†
Abstract
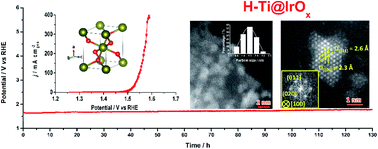
Iridium oxide (IrO2) is one of the best known electrocatalysts for the oxygen evolution reaction (OER) taking place in a strongly acidic solution. IrO2 nanocatalysts with high activity as well as long-term catalytic stability, particularly at high current densities, are highly desirable for proton exchange membrane water electrolysis (PEM-WE). Here, we report a simple and cost-effective strategy for depositing ultrafine oxygen-defective IrOx nanoclusters (1–2 nm) on a high-surface-area, acid-stable titanium current collector (H-Ti@IrOx), through a repeated impregnation–annealing process. The high catalytically active surface area resulting from the small size of IrOx and the preferable electronic structure originating from the presence of oxygen defects enable the outstanding OER performance of H-Ti@IrOx, with low overpotentials of 277 and 336 mV to deliver 10 and 200 mA cm−2 in 0.5 M H2SO4. Moreover, H-Ti@IrOx also shows an intrinsic specific activity of 0.04 mA cmcatalyst−2 and superior mass activity of 1500 A gIr−1 at an overpotential of 350 mV. Comprehensive experimental studies and density functional theory calculations confirm the important role of oxygen defects in the enhanced OER performance. Remarkably, H-Ti@IrOx can continuously catalyze the OER in 0.5 M H2SO4 at 200 mA cm−2 for 130 hours with minimal degradation, and with a higher IrOx loading, it can sustain at such a high current density for over 500 hours without significant performance decay, holding substantial promise for use in PEM-WE.


 Please wait while we load your content...
Please wait while we load your content...