An artificial photosynthetic system with CO2-reducing solar-to-fuel efficiency exceeding 20%†
Abstract
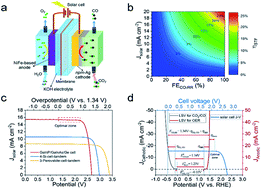
Artificial photosynthetic systems store solar energy in chemical fuels via CO2 reduction or renewable hydrogen evolution from water splitting. But only relatively moderate CO2-reducing solar-to-fuel efficiency (ηSTF < ca. 18%) was achieved previously even using the most active gold-based electrocatalyst for the CO2 reduction reaction (CO2RR), which is much lower than the current highest solar-to-hydrogen efficiency (ηSTH = 30%). Here, we report a strategy based on a monolithic nanoporous silver-based membrane electrocatalyst (npm-Ag) to increase the density of active sites while breaking the three-phase diffusion-limit length of previous membrane electrodes, improving the effective thickness of the catalyst layer from tens of nanometers to several micrometers without any polymer additive. The npm-Ag showed exceptionally selective reduction of CO2 to CO at low overpotentials (ca. 80%@40 millivolts, ca. 100%@90–290 millivolts). The catalyst also allowed a nearly full use of the photocurrent of the state-of-the-art solar cells and gave a projected maximum ηSTF of ca. 25%. Combined with a NiFe-based O2-evolving anode, a stable CO2-reducing photosynthetic system with an ηSTF of ca. 20.1% was demonstrated experimentally for 28 hours in the absence of a DC/DC converter.


 Please wait while we load your content...
Please wait while we load your content...