Chemical looping hydrogen storage and production: use of binary ferrite-spinel as oxygen carrier materials†
Abstract
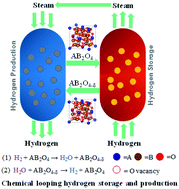
Chemical looping hydrogen storage and the recovery of iron oxides by the redox cycles were recommended as an emerging approach for large-scale hydrogen storage with a high volumetric hydrogen storage density. However, iron oxides should be operated at a high temperature (>800 °C) for its sufficient redox activity, which would lead to a rapid deterioration of hydrogen storage performance over cycles. In this work, a series of ferrite-spinel materials A0.25Fe2.75O4 (A = Co, Cu, Ni, Zn or Mn) were prepared. Among all the additives to iron oxides, Co0.25Fe2.75O4 exhibits the highest volumetric hydrogen storage density (∼62.47 g L−1) and an average hydrogen production rate (∼132 μmol g−1 min−1) under 550 °C. Besides, the storage capacity was maintained over 10 cycles. The volumetric hydrogen storage density of this material was proportionate to the most advanced Rh–FeOx containing rare-earth metal; thus, it may have the potential for industrial application.


 Please wait while we load your content...
Please wait while we load your content...