Enantioselective copper-catalysed defluorosilylation of trifluoro-methylated alkenes with silylboronates†
Abstract
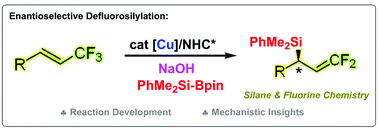
An efficient method for the construction of gem-difluoroallylsilanes with high enantiomeric excess via a copper-catalysed defluorosilylation of trifluoromethylated alkenes with silylboronates is described. The key to this high enantioselectivity is the careful selection of NaOH as the base and using a triazolium-based chiral N-heterocyclic carbene (NHC) as a ligand in the presence of a copper catalyst. The reaction conditions are mild, and excellent functional group compatibility is observed. This strategy addresses the limitations of the previously described base-mediated defluorosilylation under transition metal-free conditions, which can lead to erosion of the enantioselectivity. The synthetic utilities of the obtained gem-difluoroallylsilanes are also presented. Computational and experimental data suggest that the reaction proceeds through the enantioselective insertion of silyl-Cu/NHC species into the double bond of trifluormethyl alkenes and the Cu-mediated β-F elimination steps.
- This article is part of the themed collection: 2020 Organic Chemistry Frontiers HOT articles


 Please wait while we load your content...
Please wait while we load your content...