In situ transformation of a tridentate to a tetradentate unsymmetric Schiff base ligand via deaminative coupling in Ni(ii) complexes: crystal structures, magnetic properties and catecholase activity study†
Abstract
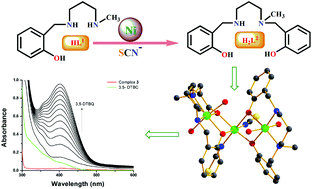
A new dimeric Ni(II) complex, [Ni2L12(CH3CN)4](ClO4)2·2CH3CN (1), was synthesized using an N2O donor reduced Schiff base [(HL1) = 2-[(3-methylamino-propylamino)-methyl]-4-phenol]. Surprisingly, during an attempt to replace its ClO4− ion with SCN−, the N2O donor ligand in situ converted to a tetradentate N2O2 donor ligand and formed a metal complex, [Ni(HL2)(NCS)(CH3CN)] (2). A probable mechanism via deaminative coupling for this conversion is proposed. Using 2 as a metalloligand under basic conditions, a trinuclear metal complex, [Ni3(L2)2(NCS)2(H2O)4]·H2O (3), was prepared. Single crystal structural characterization revealed that in all three metal complexes, the Ni(II) atoms were in an octahedral environment with coordinated solvent molecules (CH3CN in 1 and 2 and H2O in 3). Among the three metal complexes, 1 and 3 showed catecholase-like biomimicking activity. The calculation of the turnover numbers (Kcat = 7.9 for 1, 14.5 for 3) reveals that 3 is a better catalyst than 1. Mechanistic cycles are proposed for this biomimicking activity on the basis of ESI-MS spectrometry and iodometric measurements. Temperature-dependent magnetic susceptibility measurements suggest that the Ni(II) ions in metal complexes 1 and 3 are antiferromagnetically coupled (J = −32.22 cm−1 for 1, J = −10.4 cm−1 for 3), consistent with their geometries and bridging angles. Theoretically calculated J values (J = −40.15 cm−1 for 1, J = −14.53 cm−1 for 3) by the DFT method corroborate well with the experimental values.


 Please wait while we load your content...
Please wait while we load your content...