One-component rapid Norrish Type II photoinitiation of bulk photo-CuAAC polymer networks†
Abstract
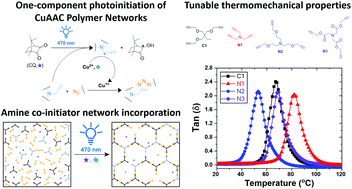
Networks formed using photo-initiated copper-catalyzed azide–alkyne cycloaddition (photo-CuAAC) polymerizations exhibit extraordinary toughness and high stability making them an ideal material for applications ranging from dental composites to membranes. The current design for photoinitiation of solvent-free CuAAC networks is accomplished under blue light using a Norrish Type II initiating system comprising camphorquinone (CQ) and a tertiary amine (TA). Although TAs are required for rapid polymerization kinetics, their presence in the network can act as a plasticizer and is often malodorous and toxic. Here, a one-component, Type II photoinitiation scheme for the photo-CuAAC polymeriziation is achieved by incorporating the TA coinitiating species into the backbone of the polymer network as a crosslinking moiety. Formulations with the polymer network-incorporated TA exhibited rapid polymerization kinetics, reaching >90% conversion with just 5 min of 470 nm irradiation at 30 mW cm−2. Varying the structure of the amine centered trialkynes allowed for tunability of the glass transition temperature from 53 to 82 °C. Moreover, only catalytic amounts of the amine-centered alkyne monomers were required to replace the external TA without adversely affecting the rapid polymerization kinetics or the thermomechanical properties of the network. Development of such monomers further demonstrates the tunability of bulk photo-CuAAC polymer network properties and expands upon their broad range of applications.


 Please wait while we load your content...
Please wait while we load your content...