A methanol–choline chloride based deep eutectic solvent enhances the catalytic oxidation of lignin into acetovanillone and acetic acid†
Abstract
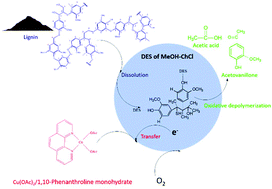
The low reactivity of lignin limits its performance in terms of selective oxidative cleavage of the C–C bond and most researchers employ lignin models as substrates for producing useful small molecules while the uses of real lignin are largely limited to low value material applications. Herein, a methanol/choline chloride (MeOH–ChCl)-based deep eutectic solvent (DES) was used to improve the solubility of lignin and the redox potential of the catalyst Cu(OAc)2/1,10-phenanthroline. A high total yield of acetovanillone and acetic acid (87.12%) was obtained from alkaline lignin under mild conditions of 60 °C for 3 h. Two-dimensional heteronuclear single-quantum coherence nuclear magnetic resonance (2D-HSQC NMR) spectra indicated that condensation and depolymerisation reactions of side chain groups of alkaline lignin occurred simultaneously, and longer side chains may inhibit acetic acid formation, while more guaiacyl units may increase the acetovanillone yield. Additionally, this catalytic oxidation system has the potential to produce acetovanillone and acetic acid from DES extracted lignin, pretreated corncob and raw materials of corncob.


 Please wait while we load your content...
Please wait while we load your content...