A pyridinium anionic ring-opening reaction applied to the stereodivergent syntheses of Piperaceae natural products†
Abstract
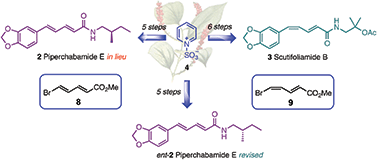
A stereodivergent strategy has been devised to access the diene motif found in biologically active compounds from the Piperaceae family. Herein the first total syntheses of 2E,4E configured piperchabamide E (2) and its enantiomer (ent-2), as well as 2E,4Z configured scutifoliamide B (3), are narrated. The mainstay in the adopted approach is the gram-scale conversion of quaternized pyridine in a practical three-step sequence to access isomerically pure conjugated bromodiene esters 2E,4E8 and 2E,4Z9 by differential crystallization. Even though the developed oxidation protocol forms the basis of the entailed divergent strategy, the geometrical integrity of the involved bromodiene motive can be controlled by the choice of solvent. Thus, while oxidation of pure bromodienal 2E,4Z7 in methanol yields equal amounts of bromodiene esters 2E,4E8 and 2E,4Z9, only bromodiene ester 2E,4Z10 is formed in isopropanol. Subseqently, capitalizing on a stereoretentive Suzuki cross-coupling and direct amidation of the corresponding esters, the featured natural products can be accessed in five and six steps, respectively. The somewhat surprising (R)-configured amine portion, which has been assigned to piperchabamide E (2), is facilitated by a Curtius rearrangement. Following this, the actual amine portion is shown to be (S)-configured.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...