Synthesis of 2-bromo- and 2-phenyl-neo-confused porphyrins†
Abstract
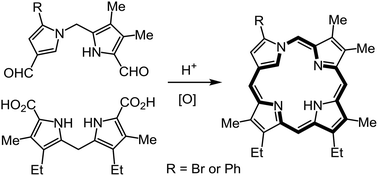
Neo-confused porphyrins (neo-CPs), porphyrin isomers with a 1,3-connected pyrrolic subunit, are aromatic structures with a CNNN coordination core. Previously, examples of neo-CPs with fused benzo units or electron-withdrawing ester substituents have been described. In this paper, two new examples of neo-CPs are reported that lack a fused aromatic unit or an ester moiety, but instead have a bromo or phenyl substituent on the neo-confused ring. Acid-catalyzed condensation of suitably substituted 1,2′-dipyrrylmethane dialdehydes with a 2,2′-dipyrrylmethane, followed by oxidation with aqueous ferric chloride solutions, afforded the neo-CPs in 40–45% yield. These porphyrin analogues had slightly reduced diatropic ring currents and slowly decomposed in solution. The related palladium(II) and nickel(II) complexes proved to be very unstable, even though the diatropicity of the macrocycle was enhanced. This study shows that stabilizing substituents are necessary for investigations into this class of porphyrinoids. Attempts to prepare imidazole versions of neo-CPs were unsuccessful.
- This article is part of the themed collection: Supramolecular chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...
