Non-hydrogen bond catalyst-mediated diastereoselective conjugate additions of 5H-oxazol-4-ones to o-hydroxyphenyl-substituted p-quinone methides†
Abstract
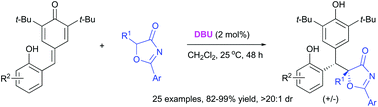
An efficient DBU-catalyzed conjugate addition of 5H-oxazol-4-ones to o-hydroxyphenyl-substituted p-quinone methides has been developed, affording the valuable diarylmethanes in high yields with excellent diastereoselectivity. This strategy demonstrates a robust access to a wide range of diarylmethane derivatives possessing biologically significant o-hydroxyphenol and p-hydroxyphenol moieties under mild reaction conditions.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...