Highly stereocontrolled total synthesis of secodolastane diterpenoid isolinearol†
Abstract
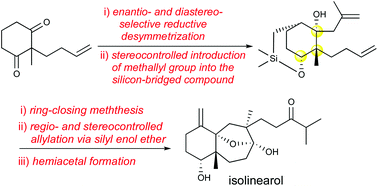
The first asymmetric total synthesis of isolinearol has been achieved with high stereoselectively. The synthetic method includes enatio- and diastereoselective reductive desymmetrization, stereocontrolled introduction of the methallyl group, regio- and stereocontrolled allylation and introduction of the side chain carbonyl group using olefin cross-metathesis with a pinacol vinyl boronic ester.


 Please wait while we load your content...
Please wait while we load your content...