Oxidative damage of proline residues by nitrate radicals (NO3˙): a kinetic and product study†
Abstract
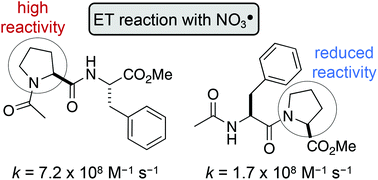
Tertiary amides, such as in N-acylated proline or N-methyl glycine residues, react rapidly with nitrate radicals (NO3˙) with absolute rate coefficients in the range of 4–7 × 108 M−1 s−1 in acetonitrile. The major pathway proceeds through oxidative electron transfer (ET) at nitrogen, whereas hydrogen abstraction is only a minor contributor under these conditions. However, steric hindrance at the amide, for example by alkyl side chains at the α-carbon, lowers the rate coefficient by up to 75%, indicating that NO3˙-induced oxidation of amide bonds proceeds through initial formation of a charge transfer complex. Furthermore, the rate of oxidative damage of proline and N-methyl glycine is significantly influenced by its position in a peptide. Thus, neighbouring peptide bonds, particularly in the N-direction, reduce the electron density at the tertiary amide, which slows down the rate of ET by up to one order of magnitude. The results from these model studies suggest that the susceptibility of proline residues in peptides to radical-induced oxidative damage should be considerably reduced, compared with the single amino acid.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...