Synthesis of paenipeptin C′ analogues employing solution-phase CLipPA chemistry†
Abstract
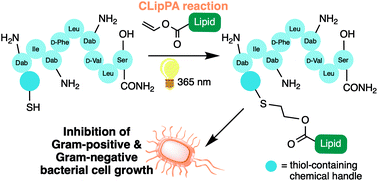
We herein report the synthesis of analogues of the antimicrobial lipopeptide, paenipeptin C′, by installing varying lipid moieties using thiol–ene CLipPA (Cysteine Lipidation on a Peptide or Amino Acid) chemistry. Biological evaluation against both Gram-negative and Gram-positive strains indicated that several analogues possessed potent broad-spectrum antimicrobial activity.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...