Enantioselective synthesis and determination of the absolute configuration of the male sex pheromone of the parasitoid wasp Urolepis rufipes†
Abstract
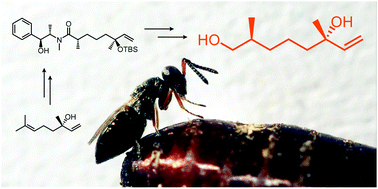
Males of the parasitoid wasp Urolepis rufipes use 2,6-dimethyl-7-octene-1,6-diol as a sex pheromone to attract virgin females. Herein, we determine the absolute configuration of the pheromone to be (2S,6S)-2,6-dimethyl-7-octene-1,6-diol (2S,6S-6) and present a stereoselective synthesis of the natural enantiomer of this new linalool derivative. In addition, we show that female wasps respond to the natural 2S,6S-6 stereoisomer while 2R,6S-6 is behaviorally inactive.


 Please wait while we load your content...
Please wait while we load your content...