The quasi-irreversible inactivation of cytochrome P450 enzymes by paroxetine: a computational approach†
Abstract
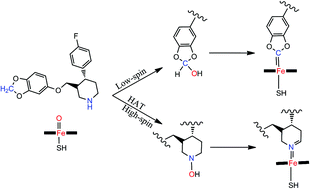
The mechanism-based inactivation (MBI) of P450 by paroxetine was investigated by computational analysis. The drug–enzyme interactions were figured out through studying energy profiles of three competing mechanisms. The potency of paroxetine as P450's inhibitor was estimated based on the availability of two active sites for the MBI in the paroxetine structure. The inactivation by the amino site of paroxetine mainly proceeds via the hydrogen atom transfer pathway because of the lower energy demand of its rate determining step. In addition, the low-spin state is the predominant route in the MBI at the methylenedioxo active site as a result of being rebound barrier-free mechanism. Our comparative investigation showed that inactivation at the secondary amine is thermodynamically more favorable because of the lower energy barrier of the dehydration mechanism of the hydroxylated paroxetine complex than its methylenedioxo counterpart. The results of docking analysis coincided with the outputs of DFT calculations since the docking pose with the lowest binding affinity is that for conformation with polar interaction between the amino group of paroxetine and the oxo moiety of P450's active site. Assessment of the molecular dynamics simulations trajectories revealed the favorable interaction of paroxetine with P450.
- This article is part of the themed collections: Chemical Biology in OBC and Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...