Catalyst and solvent switched divergent C–H functionalization: oxidative annulation of N-aryl substituted quinazolin-4-amine with alkynes†
Abstract
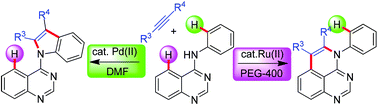
The development of site-selective C–H functionalizations/annulations is one of the most challenging practices in synthetic organic chemistry particularly for substrates bearing several similarly reactive C–H bonds. Herein, we describe catalyst and solvent controlled ortho/peri site-selective oxidative annulation of C–H bonds of N-aryl substituted quinazolin-4-amines with internal alkynes. The ortho C–H selective annulation was observed using Pd-catalyst in DMF to give indole-quinazoline derivatives, while, Ru-catalyst in PEG-400 favoured the peri C–H bond annulation exclusively to furnish pyrido-quinazoline derivatives.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...