Establishing linear-free-energy relationships for the quadricyclane-to-norbornadiene reaction†
Abstract
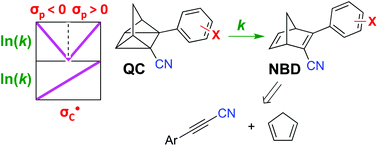
The kinetics of the thermal quadricyclane-to-norbornadiene (QC-to-NBD) isomerization reaction was studied for a large selection of derivatives where the one NBD double bond contains a cyano and aryl substituent of either electron-withdrawing or -donating character. While the kinetics data did not satisfy a linear-free-energy-relationship for all the derivatives based on Hammett σ values, we found individual linear relationships for derivatives containing either electron-withdrawing or electron-donating para substituents on the aryl group; with the most electron-witdrawing substituent in the one series and with the most electron-donating substituent in the other providing the fastest reaction (corresponding to opposite slopes of the Hammett plots). All data were well described, however, by a linear relationship when using Creary radical 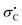 values; the correlation could be slightly improved by using a combination of σ and
values; the correlation could be slightly improved by using a combination of σ and 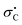 values (used in ratio of 0.104 : 1). The results imply a combination of polar and free radical effects for the isomerization reaction of this specific class of derivatives, with the latter playing the most significant role. The NBD derivatives were prepared by Diels–Alder cycloaddition reactions between cyclopentadiene and 3-arylpropiolonitriles, and in the case of bromophenyl derivatives further cyanation and Sonogashira coupling reactions were performed.
values (used in ratio of 0.104 : 1). The results imply a combination of polar and free radical effects for the isomerization reaction of this specific class of derivatives, with the latter playing the most significant role. The NBD derivatives were prepared by Diels–Alder cycloaddition reactions between cyclopentadiene and 3-arylpropiolonitriles, and in the case of bromophenyl derivatives further cyanation and Sonogashira coupling reactions were performed.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...