Formal enantioselective synthesis of nhatrangin A†
Abstract
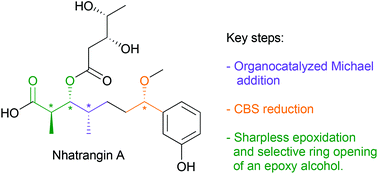
A new and straightforward synthesis of the C1–C7 core fragment of nhatrangin A was achieved in 14 steps from achiral 3-hydroxybenzaldehyde, without the need of chiral reagents or enzymatic resolution to introduce the chiral centers. The key asymmetric steps include in particular a highly enantioselective organocatalyzed Michael addition on an aryl vinyl ketone, a Sharpless asymmetric epoxidation and a subsequent regioselective ring opening of the resulting chiral epoxide. This work represents the first formal enantioselective synthesis of nhatrangin A.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...