Na-Ion storage in iron hydroxide phosphate hydrate through a reversible crystalline-to-amorphous phase transition†
Abstract
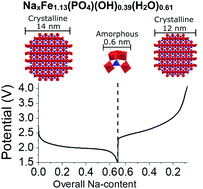
Iron(III) hydroxide phosphate hydrate Fe1.13(PO4)(OH)0.39(H2O)0.61 is investigated for the first time as a Na-ion battery cathode, which reveals that the material exhibits similar storage capacities for Na- and Li-ions at relatively low current rates (i.e. C/10). Interestingly, operando X-ray diffraction shows that insertion of Na-ions induces a solid solution transition in the crystalline Fe1.13(PO4)(OH)0.39(H2O)0.61 end-member simultaneously with a major amorphization. This result adds to the series of observations of phosphate-based materials undergoing order-disorder transitions during Na-ion storage. Fe1.13(PO4)(OH)0.39(H2O)0.61 is thus ideal for enhancing our knowledge on such phenomena. To this end, using total X-ray scattering with pair distribution function analysis, we show that the amorphous phase is Na-rich NaxFe1.13(PO4)(OH)0.39(H2O)0.61 with the local [FeO6]-[PO4] motif retained but with coherence lengths of only ca. 0.6 nm. Our investigation also reveals that the crystallinity of Fe1.13(PO4)(OH)0.39(H2O)0.61 is regained upon Na-extraction (battery recharge), i.e. the order-disorder transition is reversible.
- This article is part of the themed collection: Spectroscopy and scattering for chemistry


 Please wait while we load your content...
Please wait while we load your content...