Two-dimensional composite of D-Ti3C2Tx@S@TiO2 (MXene) as the cathode material for aluminum-ion batteries†
Abstract
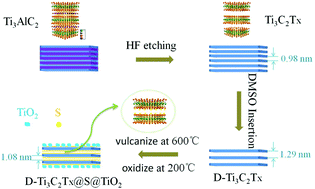
MXenes, the two-dimensional layered materials, are widely used in electrochemical storage devices and exhibit excellent electrochemical performances. Aluminum-ion batteries are favored by researchers around the world due to our urgent need for emerging clean energy; the study of cathode materials is most important in aluminum-ion battery research. Herein, we have prepared a two-dimensional layered composite of D-Ti3C2Tx@S@TiO2 by a simple method, which shows excellent electrochemical performance in aluminum-ion batteries. The discharge specific capacity is 151.3 mA h g−1 after 120 cycles, which is about 80.0 mA h g−1 higher than that of Ti3C2Tx in aluminum-ion batteries, and the capacity retention rate is 72.3%. The Coulomb efficiency is about 85% during steady cycling. The reasons for its excellent electrochemical performances are explored herein, and it is proved by DFT calculations that Ti3C2S is more stable than Ti3C2OH in aluminum-ion batteries. The elemental sulfur reacted with the exposed Ti-ions to form some Ti–S bonds, which play a supporting role in maintaining the original structure of the material and preventing the superposition of layers between the materials. Some TiO2 nanoparticles were grown in situ on the surface of the material by further oxidation treatment, which improved the stability of the material. In addition, we studied the charge/discharge mechanism of the aluminum-ion battery using Ti3C2Tx as the cathode material based on the changes in the contents of Al and Cl, and the change in the valence of Ti-ions. The results show that [AlCl4]− was intercalated/de-intercalated in the layers of the cathode material during the charge/discharge processes.


 Please wait while we load your content...
Please wait while we load your content...