Stepwise ammonium enrichment using selective battery electrodes†
Abstract
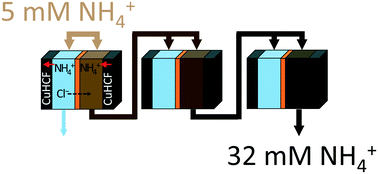
Ammonium is typically removed from treated wastewaters before discharge by converting it to nitrogen gas, but its capture and reuse could provide a new strategy for energy recovery at treatment plants. A three-stage electrochemical approach was developed here to selectively remove and concentrate ammonium derived from wastewater. Each stage contained a battery electrode deionization (BDI) cell containing two copper hexacyanoferrate (CuHCF) electrodes separated into two channels using an anion exchange membrane. Through application of a low applied voltage (0.3 V) in each of the three stages, ammonium was concentrated greater than 6 times, from 5 to 32 mM (90 to 576 mg L−1), with minimal changes in the concentration of other cations (Na+, K+, Mg2+, and Ca2+) present in the water due to the high ammonium ion selectivity of CuHCF electrodes under these operating conditions. The cumulative energy use for the three-stage process was only 2.0 kW h per kg-N, compared to the 14 kW h per kg-N that would be needed to manufacture this amount of ammonium from nitrogen gas. Nitrogen recovery methods such as these will be needed to further transform used water plants into more effective resource recovery treatment plants.


 Please wait while we load your content...
Please wait while we load your content...