Effective removal and selective capture of copper from salty solution in flow electrode capacitive deionization†
Abstract
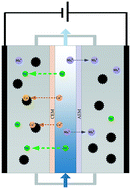
Heavy metal removal and recovery from industrial wastewaters have aroused great concern. However, selective concentration of these target ions remains a big challenge given the high concentration of interfering ions like Na+. In this study, treatment of salty influents containing copper ions was carried out by flow-electrode capacitive deionization (FCDI) for the removal and recovery of copper. A variety of operation conditions were investigated including the operation mode of the flow electrode, applied voltage, initial pH of the flow electrode and long-term operation. Results showed that separation was achieved in the electrode chamber, since Cu2+ was preferentially deposited or adsorbed on the carbon particles while Na+ was mostly distributed in the electrolyte. This phenomenon was more evident in a short-circuited closed cycle (SCC) mode due to strong Na+ desorption. The removal efficiency of Cu2+ escalated consistently with the increase of applied voltage, and the removed Cu2+ remained in the carbon particles. The formation of Cu species could be regulated by pH adjustment, and X-ray photoelectron spectroscopy (XPS) analysis confirmed that the low initial pH of the flow electrode contributed to the production of Cu0. The Cu2+ removal performance retained ∼94% of the initial value while for Na+ only ∼90% was retained after 24-hour operation, which was related to the co-ion leakage phenomenon; the maximum loading content reached ∼5 mg Cu per g AC.
- This article is part of the themed collection: Capacitive deionisation and electrosorption 2020


 Please wait while we load your content...
Please wait while we load your content...