Oxidation and adsorption of antimony(iii) from surface water using novel Al2O3-supported Fe–Mn binary oxide nanoparticles: effectiveness, dynamic quantitative mechanisms, and life cycle analysis†
Abstract
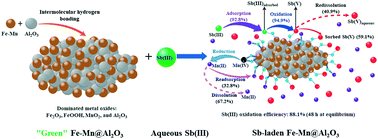
Antimony(III) or Sb(III) contamination in surface waters poses a serious threat to the ecological system and human health, and green and cost-effective technologies are urgently needed to mitigate its toxic effects. We green synthesized a novel oxidative sorbent, referred to as Al2O3-supported Fe–Mn binary oxide nanoparticles (Fe–Mn@Al2O3), and investigated its removal effectiveness and dynamic quantitative removal mechanisms of Sb(III) at environmentally relevant levels from simulated surface water. Fe–Mn binary oxides (Fe–Mn) were successfully attached on the surface of Al2O3via intermolecular hydrogen bonding, resulting in the formation of Fe–Mn@Al2O3 with a larger specific surface area, greater oxidizing reactivity, and more sorption sites compared to Fe–Mn. The resulting composite is mainly composed of FeOOH, Fe2O3, MnO2, and Al2O3. Life cycle assessment (LCA) indicated that the Fe–Mn@Al2O3 synthesis met green chemistry principles. Fe–Mn@Al2O3 at a Fe : Al2O3 molar ratio of 1 : 2 displayed an enhanced Sb(III) sorption capacity of 272.2 mg g−1 at pH 6.4 within 48 h. Sorption kinetic data were adequately simulated with the pseudo second-order kinetic model and the intraparticle diffusion model, suggesting that the sorption kinetics were a combination of chemisorption and intraparticle diffusion. The Freundlich isotherm model outperformed the Langmuir model in simulating the sorption isotherm data, which aligns with the heterogeneous surface sorption sites of Fe–Mn@Al2O3. MnO2 in Fe–Mn@Al2O3 oxidized Sb(III) to Sb(V), whereas FeOOH and Al2O3 acted as adsorption sites towards Sb(III) and Sb(V). Most importantly, Fe–Mn@Al2O3 still maintained high sorption efficiencies towards Sb(III) after five consecutive regeneration cycles. The dynamic quantitative removal mechanisms were proposed thereafter. Upon equilibrium, 92.8% of Sb(III) was sorbed by Fe–Mn@Al2O3, and 94.9% of sorbed Sb(III) was oxidized to produce Sb(V) and Mn(II). 59.1% of the formed Sb(V) was adsorbed onto Fe-Mn@Al2O3 and 40.9% of Sb(V) was released into the solution. The new quantitative and mechanistic insights contribute to an improved understanding of the uptake of Sb(III) by Fe–Mn@Al2O3 in natural and engineered systems, and the results may guide further preparation and application of reactive adsorbents for removal of redox-active contaminants from water.
- This article is part of the themed collection: Environmental Remediation


 Please wait while we load your content...
Please wait while we load your content...