Copper oxide nanoparticle dissolution at alkaline pH is controlled by dissolved organic matter: influence of soil-derived organic matter, wheat, bacteria, and nanoparticle coating†
Abstract
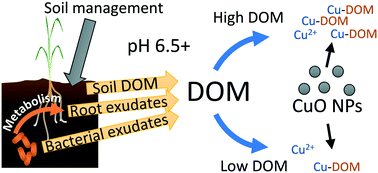
Dissolution of CuO nanoparticles, releasing Cu ions, is a primary mechanism of Cu interaction in the rooting zone of plants. CuO dissolution is sometimes incorrectly considered negligible at high pH, since complexation of Cu with dissolved organic matter may enhance nanoparticle dissolution. Therefore data on the effects of plant-microbial-soil interactions on nanoparticle dissolution, particularly in alkaline soils, are needed. Dissolution of CuO nanoparticles (100 mg kg−1 Cu) was studied in sand supplemented with factorial combinations of wheat growth, a root-colonizing bacterium, and saturated paste extracts (SPEs) from three alkaline, calcareous soils. In control sand systems with 3.34 mM Ca(NO3)2 solution, dissolved Cu was low (266 μg L−1 Cu). Addition of dissolved organic matter via wheat root metabolites and/or soil SPEs increased dissolved Cu to 795–6250 μg L−1 Cu. Dissolution was correlated with dissolved organic carbon (R = 0.916, p < 0.0001). Ligands >3 kDa, presumably fulvic acid from the SPEs, complexed Cu driving solubility; the addition of plant exudates further increased solubility 1.5–3.5×. The root-colonizing bacterium decreased dissolved Cu in sand pore waters from planted systems due to metabolism of root exudates. Batch solubility studies (10 mg L−1 Cu) with the soil SPEs and defined solutions containing bicarbonate or fulvic acid confirmed elevated CuO nanoparticle solubility at >7.5 pH. Nanoparticle dissolution was suppressed in batch experiments compared to sand, via nanoparticle organic matter coating or homoconjugation of dissolved organic matter. Alterations of CuO nanoparticles by soil organic matter, plant exudates, and bacteria will affect dissolution and bioavailability of the CuO nanoparticles in alkaline soils.


 Please wait while we load your content...
Please wait while we load your content...
