Formation of Fe(iii)–As(v) complexes: effect on the solubility of ferric hydroxide precipitates and molecular structural identification†
Abstract
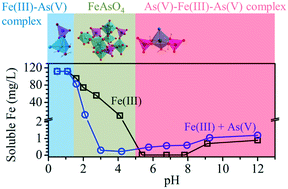
Ferric iron (Fe(III)) and arsenate (As(V)) generally co-exist in natural waters. Also, Fe(III) chemicals are widely used for treatment of As(V) in water. This paper reported the existence of Fe(III)–As(V) complexes, which are molecular clusters containing only one iron atom with sizes of less than 1 nm, typically considered as soluble species. The formation of Fe(III)–As(V) complexes varies with the change of pH. Specifically, the soluble Fe(III)–As(V) complex formed at acidic conditions (pH <2) with a Fe : As mole ratio of 1 : 1. When the pH increased to 2–5, Fe(III) and As(V) formed precipitate rather than a soluble complex, which led to a reduced solubility of Fe(III) at pH 2–5. The soluble Fe(III)–As(V) complex again appeared at pH >5 with the Fe : As mole ratio of 1 : 2. DFT calculation identified that these soluble Fe(III)–As(V) complexes were in a monodentate configuration. The chloride was found to be coordinated with the Fe(III)–As(V) complexes, which improved their thermodynamic stability. The existence of these soluble complexes promoted the solubility of Fe(III) at neutral pH (i.e., total soluble Fe(III) increased to 0.73 mM (∼41 mg L−1) with an initial 0.1 M Fe(III)). The findings in this study shed new light on the understanding of the transport and fate of Fe(III) and As(V) in natural water systems, as well as the As(V) leaching from the sludge produced in the Fe(III)-based As(V) removal techniques.
- This article is part of the themed collection: Environmental Science: Nano Cover Art


 Please wait while we load your content...
Please wait while we load your content...