Selective catalytic oxidation of ammonia over nano Cu/zeolites with different topologies†
Abstract
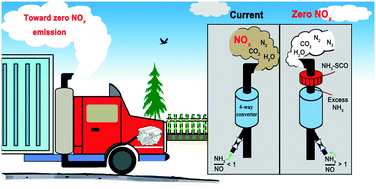
The selective catalytic oxidation of ammonia (NH3-SCO) is the last mitigation step in exhaust treatment using a 4-way catalytic converter to convert any excess and unreacted NH3 (that was used as a reductant of NOx) into environmentally benign N2 and H2O. Here, we report a series of highly reactive and selective nano Cu/zeolites for the NH3-SCO reaction. The NH3-SCO activity was found in the order nano Cu/ZSM-5 (MFI topology) > Cu/Beta (BEA) > Cu/MCM-49 (MWW) > Cu/Y (FAU) > Cu/Mordenite (MOR) > Cu/Ferrierite (FER). The best catalyst, i.e., nano Cu/ZSM-5, achieves 98% NH3 conversion at 250 °C with the N2 yield maintained at >98% even at up to 500 °C. When assessed under practical exhaust conditions in the presence of moisture (5% H2O) as well as that after hydrothermal aging (5% H2O, 850 °C, 8 h), the nano Cu/ZSM-5 exhibited only minor deactivation as a result of its good retention of Cu dispersion, pore structure and specific surface area. Furthermore, small micropore (10-membered ring, 10-MR) topologies were found to be crucial in maintaining high N2 yields. For Cu/Y and Cu/Mordenite, composed of 12-MR pores that are non-interconnected with smaller pores, their N2 yields were compromised by forming NOx at temperatures above 400 °C. Based on the in situ DRIFTS study, the iSCR mechanism appears to be applicable for all fresh and aged Cu/zeolites with the exception of fresh Cu/MCM-49 that follows the imide mechanism.
- This article is part of the themed collection: Nanomaterials in air


 Please wait while we load your content...
Please wait while we load your content...