Effects of pH and electrolytes on the sheet-to-sheet aggregation mode of graphene oxide in aqueous solutions†
Abstract
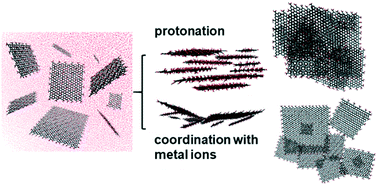
In this work, the aggregation mode of graphene oxide (GO) was identified. Atomic force microscopy (AFM) characterization indicated that protonation at lower pH was more efficient in accelerating the stacking of GO than binding with metal ions, and molecular dynamics (MD) simulations revealed that this should be attributed to the different favorable aggregation modes under different aqueous chemistries. GO tended to aggregate in the face-to-face or partial face-to-face mode at lower pH, and in the partial face-to-face, edge-to-edge, or point-to-point mode at higher pH with the presence of metal ions. To elucidate the mechanisms where pH and metal ions can exert distinct effects on the aggregation mode of GO, density functional theory calculations were performed. The results demonstrated that the favorable face-to-face and partial face-to-face aggregation at lower pH was mainly guided by the stronger π–π interaction and suppressed water-mediated steric hindrance; however, hydrogen bond and vdW interactions were not important in determining the aggregation mode. Overall, the distinct aggregation mode of GO under different aqueous chemistries will shed light on predicting the toxicity and environmental applications of GO.
- This article is part of the themed collections: Environmental Remediation and Environmental fate of nanomaterials


 Please wait while we load your content...
Please wait while we load your content...
