Emerging investigator series: first-principles and thermodynamics comparison of compositionally-tuned delafossites: cation release from the (001) surface of complex metal oxides†
Abstract
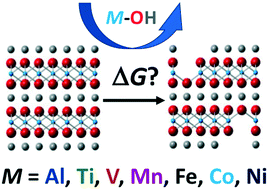
Nanoscale complex metal oxides have transformed how technology is used around the world. A ubiquitous example is the class of electroreactive cathodes used in Li-ion batteries, found in portable electronics and electric cars. Lack of recycling infrastructure and financial drivers contribute to improper disposal, and ultimately, introduction of these materials into the environment. Outside of sealed operational conditions, it has been demonstrated that complex metal oxides can transform in the environment, and cause negative biological impact through leaching of cations into aqueous phases. Using a combined DFT and thermodynamics methodology, insights into the mechanism and driving forces of cation release can be studied at the molecular-level. Here, we describe design principles that can be drawn from previous collaborative research on complex metal oxide dissolution of the Li(NiyMnzCo1−y−z)O2 family of materials, and go on to posit ternary complex metal oxides in the delafossite structure type with controlled release behavior. Using equistoichiometric formulations in the delfossite structure, we use DFT and thermodynamics to model cation release. The release trends are discussed in terms of lattice stability, solution chemistry/solubility limits, and electronic/magnetic properties. Intercalation voltages are calculated and discussed as a predictive metric for potential functionality of the model materials.
- This article is part of the themed collection: Emerging Investigators Series

 Please wait while we load your content...
Please wait while we load your content...
