Effect of the chlorine substitution position of the end-group on intermolecular interactions and photovoltaic performance of small molecule acceptors†
Abstract
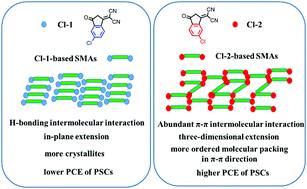
The structure–property relationships of small molecular acceptors (SMAs) are a key issue in the molecular design of new-generation acceptor materials for further improving the device efficiencies of polymer solar cells (PSCs). Herein, three couples of SMA isomers were synthesized, based on three central fused ring units and two 1,1-dicyanomethylene-3-indanone (IC) isomer electron-withdrawing terminal units with chlorine substitutions in different positions of its benzene ring: Cl-1 with chlorine on the same side as a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of IC and Cl-2 with chlorine on the same side as the CN groups of IC. Through systematical investigation, we found that the chlorine substitution position of the terminal groups has a regular and significant influence on the molecular packing and photovoltaic performance of the SMAs. The molecular packing behavior of the SMAs is closely related to and determined by the configuration of their terminal groups, no matter which central fused ring of the SMAs is used. In particular, the Cl-1-based SMAs possess a stronger crystallinity with long range ordering packing in their molecular plane direction, while the more abundant and stereoscopic π–π intermolecular interaction in the Cl-2-based SMAs promotes the molecules to form three-dimensional charge transporting channels and leads to their red-shifted absorption and higher electron mobilities. Therefore, the Cl-2-based PSCs exhibit a higher power conversion efficiency (PCE) compared to that of the Cl-1-based devices, and the best PCE of a Cl-2 SMA-based PSC reached 16.42%. These results highlight the importance of the investigation of intermolecular interactions, packing and the arrangement of the SMAs in the solid-state, which may provide direct insights for exploring the relationship between the molecular structure and property of the photovoltaic materials. Moreover, we envision that if fragments such as end groups or side chains with more diverse molecular interactions are added into the design and the subsequent synthesis of the SMAs, this may be beneficial to promoting molecular π–π accumulation and further improving the molecular order, forming suitable molecular packing and morphology in the resulting blend films, and finally affecting the efficiency of the PSCs.
O group of IC and Cl-2 with chlorine on the same side as the CN groups of IC. Through systematical investigation, we found that the chlorine substitution position of the terminal groups has a regular and significant influence on the molecular packing and photovoltaic performance of the SMAs. The molecular packing behavior of the SMAs is closely related to and determined by the configuration of their terminal groups, no matter which central fused ring of the SMAs is used. In particular, the Cl-1-based SMAs possess a stronger crystallinity with long range ordering packing in their molecular plane direction, while the more abundant and stereoscopic π–π intermolecular interaction in the Cl-2-based SMAs promotes the molecules to form three-dimensional charge transporting channels and leads to their red-shifted absorption and higher electron mobilities. Therefore, the Cl-2-based PSCs exhibit a higher power conversion efficiency (PCE) compared to that of the Cl-1-based devices, and the best PCE of a Cl-2 SMA-based PSC reached 16.42%. These results highlight the importance of the investigation of intermolecular interactions, packing and the arrangement of the SMAs in the solid-state, which may provide direct insights for exploring the relationship between the molecular structure and property of the photovoltaic materials. Moreover, we envision that if fragments such as end groups or side chains with more diverse molecular interactions are added into the design and the subsequent synthesis of the SMAs, this may be beneficial to promoting molecular π–π accumulation and further improving the molecular order, forming suitable molecular packing and morphology in the resulting blend films, and finally affecting the efficiency of the PSCs.
- This article is part of the themed collection: Special issue in honour of Seth Marder


 Please wait while we load your content...
Please wait while we load your content...
