Metal promoted conversion of aromatic amines to ortho-phenylenediimine derivatives by a radical coupling path†
Abstract
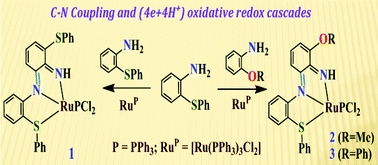
A radical path for the conversion of o-substituted arylamines to o-phenylenediimine derivatives is reported. In the presence of [RuII(PPh3)3Cl2] (RuP), 2-(phenylthio)aniline (LSNH2) acts as an o-amination agent. Reaction of LSNH2 with RuP in toluene promotes (4e + 4H+) oxidative dimerization affording an o-phenylenediimine complex of ruthenium(II). Similarly, intermolecular coupling between LSNH2 and other arylamines has been achieved.


 Please wait while we load your content...
Please wait while we load your content...