Oxamusplatin: a cytotoxic Pt(ii) complex of a nitrogen mustard with resistance to thiol based sequestration displays enhanced selectivity towards cancer†
Abstract
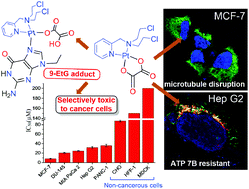
Pt(II) drugs and nitrogen mustards show severe side effects, poor tumour selectivity and face growing resistance by cancer cells due to sequestration by thiol-containing molecules (viz. glutathione (GSH) and copper ATPases like ATP7A/7B). ATP7A and ATP7B-sequestered Pt(II) complexes show dose inefficacy and resistance. The incorporation of bulky ligands and chelating leaving groups may prevent deactivation by thiols. In this work, we have synthesised four new Pt(II) complexes (3–6) of two carrier ligands, bis(2-hydroxyethyl)pyridylmethylamine (L1) and bis(2-chloroethyl)pyridylmethylamine (L2) with oxalato and cyclobutanedicarboxylato leaving groups. Among these four new complexes, the Pt(II) complex of L2 with the oxalato leaving group (5, termed “oxamusplatin”) is cytotoxic. Oxamusplatin is more resistant than cisplatin or oxaliplatin towards hydrolysis, thiol binding and sequestration by ATP7B. It targets cellular DNA and is capable of disrupting the microtubule network in the cytoskeleton. Oxamusplatin demonstrates better selectivity than oxaliplatin towards cancerous cells. It is ca. 4–10 times more cytotoxic towards metastatic prostate carcinoma (DU-145, IC50 = 21 ± 1 μM) and ca. 10–24 times more cytotoxic towards breast adenocarcinoma (MCF-7, IC50 = 8.1 ± 0.8 μM) compared to the three noncancerous cells investigated.


 Please wait while we load your content...
Please wait while we load your content...