Methanol oxidative dehydrogenation and dehydration on carbon nanotubes: active sites and basic reaction kinetics†
Abstract
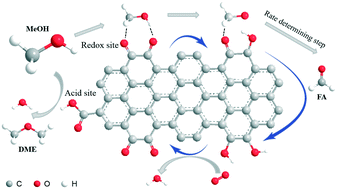
Methanol dehydrogenation to formaldehyde or dehydration to dimethyl ether are high-value-added industrial processes. Here, we applied oxidized multi-walled carbon nanotubes (oCNTs) in a methanol conversion reaction in the presence of oxygen. oCNT catalysts exhibit a methanol conversion of 60% under gentle reaction conditions, yielding DME (65%) and FA (30%) as the main products, and long term stability for over 150 h is achieved. The catalytic performance of oCNTs is even comparable to industrial metal catalysts. An in situ site titration experiment revealed that carboxyl groups were the active sites for the formation of dimethyl ether. Small organic molecules as model catalysts suggested that quinoidic carbonyl groups may be responsible for methanol ODH producing formaldehyde, while both quinoidic and ketonic carbonyl groups are responsible for total oxidation to carbon dioxide. Basic reaction kinetics and mechanisms such as reaction orders, apparent activation energy, rate determining step, etc. for the oxidative dehydrogenation and dehydration of methanol were studied systematically. A unique gentle redox catalytic activity of oCNT catalysts could be realized to reduce the overoxidation losses.


 Please wait while we load your content...
Please wait while we load your content...