Mechanism and malleability of glucose dehydration to HMF: entry points and water-induced diversions†
Abstract
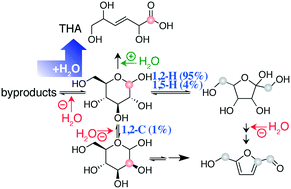
The stoichiometric dehydration of glucose to 5-hydroxymethylfurfural (HMF) converts an abundant substrate to a versatile chemical. HMF formation can be optimised by using suitable solvents including ionic liquids and DMSO, and by cosolvents such as water. A prerequisite for efficient glucose influx into pathways to HMF is the isomerization of glucose to a ketose, typically the Lewis acid catalysed conversion to fructose. Here, solvent control of the influx of glucose into pathways to HMF is mapped through isotope tracing assays and through kinetic in situ observations. Diversions from the path to HMF in the presence of water are described for the popular CrCl3/DMSO system. Addition of water to this system favors the formation of a useful byproduct instead of a mixture of inert compounds. The water-enabled formation of this product is observed for a variety of catalytsts and solvents.
- This article is part of the themed collection: 2020 Catalysis Science & Technology Hot Articles


 Please wait while we load your content...
Please wait while we load your content...