The unfolding transition state of ubiquitin with charged residues has higher energy than that with hydrophobic residues†
Abstract
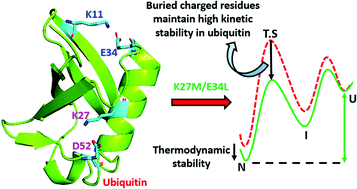
The native-state structure and folding pathways of a protein are encoded in its amino acid sequence. Ubiquitin, a post-translational modifier, primarily noted for its role in intracellular protein degradation, has two salt bridges: one relatively exposed (SB1:K11–E34) and the other relatively buried (SB2:K27–D52). Here, we study the role of hydrophobic interactions and sequence specificity in protein folding, by mutating the salt-bridge residues in ubiquitin with hydrophobic residues. Equilibrium chemical denaturation using GdnHCl shows that the SB1 null variant is thermodynamically stabilised whereas the SB2 null variant is destabilised only slightly. The thermodynamic stability of the double salt-bridge (DB) null variant is an additive effect of the individual salt bridges. Kinetic experiments show that all the salt-bridge null variants fold through a more stable intermediate with relatively faster folding rates than the wild-type. The SB2 null variant has a highly stabilised unfolding transition state (TS) and a slightly destabilised native state, leading to its kinetic instability, whereas the kinetic stability of the SB1 null variant is not compromised as its TS and native state are stabilized to a similar extent. The TS stabilisation is also additive for the DB null variant, which has the most stabilised TS and high kinetic instability. Our results underscore the importance of kinetic stability in optimising the protein energy landscape. Our study establishes the fact that the TSs can be stabilized by hydrophobic residues in the place of buried charged residues. It further highlights the role of charged residues in the protein interior in dictating the folding pathway.


 Please wait while we load your content...
Please wait while we load your content...