Improving phase-transfer catalysis by enhancing non-covalent interactions†
Abstract
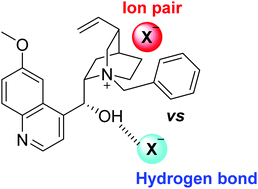
A wide variety of asymmetric transformations catalysed by chiral catalysts have been developed for the synthesis of valuable organic compounds in the past several decades. Within the asymmetric catalysis field, phase-transfer catalysis has been recognized as a powerful method for establishing useful procedures for organic synthesis. In the present study intermolecular interactions between a well-known alkaloid quinine-derived phase transfer catalyst and four different anions were characterised, analysing the competition between the pure ion-pair interaction and the intermolecular hydrogen bond established upon complexation. Finally, a theoretical study of the free-energy profile corresponding to the enantioselective conjugate cyanation of an α,β-unsaturated ketone in the presence of two different catalysts was performed.
- This article is part of the themed collections: #RSCPoster Conference and PCCP Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...