Spontaneous liquid water dissociation on hybridised boron nitride and graphene atomic layers from ab initio molecular dynamics simulations†
Abstract
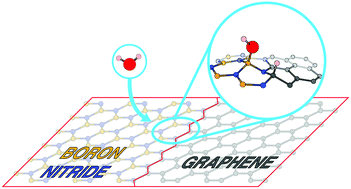
Two-dimensional materials such as graphene (G) and hexagonal boron nitride (BN) have demonstrated potential applications in membrane science and in particular for the harvesting of blue energy. Although pure G and BN atomic layers are known to remain inert towards neutral water, one may wonder about the aqueous reactivity of hybridized monolayers formed by joining BN and G sheets in a planar fashion. Here, we perform ab initio molecular dynamics calculations of liquid water in contact with all possible planar heterostructures. Remarkably, we could observe the spontaneous chemisorption and dissociation of the interfacial water molecule into its self-ions at one specific and non-standard one-dimensional border. Our simulations predict that this type of heterostructure is prone to ionize liquid water in the absence of any electrical gating.
- This article is part of the themed collection: Frontiers in Molecular Simulation of Solvated Ions, Molecules and Interfaces


 Please wait while we load your content...
Please wait while we load your content...