Pyridinium modification of a hexaazaphenalene skeleton: structure and spectroelectrochemical analysis†
Abstract
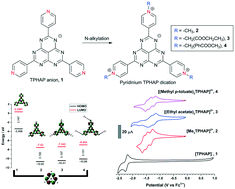
N-Alkylation of tris(4-pyridyl) hexaazaphenalene (TPHAP) anions afforded corresponding pyridinium derivatives. These compounds typically existed in their dicationic states and exhibited significantly enhanced π–π interactions within stacked dimers due to a change in charge distribution induced by the pyridinium moieties. This behaviour was attributed to the reduction in the anion–anion repulsion between the HAP cores. In addition, the presence of substituted alkyl pyridinium groups and corresponding counter anions modified the molecular packing of these derivatives. Molecular orbital calculations of the pyridinium analogues revealed close similarity of their HOMO and LUMO to the unsubstituted TPHAP with significantly lowered energies. Solution-state electrochromism was investigated by in situ spectroelectrochemical analysis. The electrochemical reduction was accompanied by the appearance of new absorption bands in the visible and NIR ranges.


 Please wait while we load your content...
Please wait while we load your content...