New uranium(vi) and isothiouronium complexes: synthesis, crystal structure, spectroscopic characterization and a DFT study†
Abstract
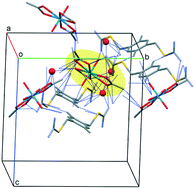
The crystal structures of S,S′-2,5-dimethylbenzene-1,4-diylbis(methylisothiouronium) diacetate (1_ac), S,S′-2,5-dimethylbenzene-1,4-diylbis(methylisothiouronium) dichloride (1_Cl), 1_U complex, S,S′-naphthalene-1,4-diylbis(methylisothiouronium) dichloride (2_Cl), and 2_U complex were determined for the first time. The supramolecular structures of the compounds obtained are mainly based on hydrogen bonding and ionic interactions between the isothiouronium cations and the counter anions Cl−, CH3COO−, and [UO2(CH3COO)3]−. Their structural and spectroscopic properties were studied, and the results of DFT calculations were compared with the experimental findings to provide insights into the properties of these new compounds. The DFT calculations indicate a strong preference for the formation of the outer-sphere complex (ion-pair) between the uranyl species and an isothiouronium cation, and very large stabilization energies of the interactions, which can be utilized for the selective binding of U(VI). The compounds obtained are the first f-element and isothiouronium salt complexes described so far.


 Please wait while we load your content...
Please wait while we load your content...