Microwave assisted slurry conversion crystallization for manufacturing of new co-crystals of sulfamethazine and sulfamerazine†
Abstract
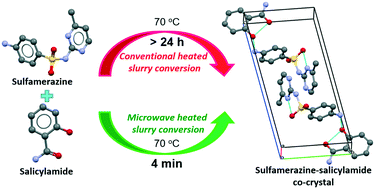
Two sulfa drugs, sulfamethazine and sulfamerazine, have been used as model compounds in the evaluation of microwave assisted slurry conversion crystallization for manufacturing of co-crystals. It is shown that the formation of the co-crystals is much faster using microwaves than simple conductive/convective heating to the same temperature. The favourable effect of using microwaves goes beyond the simple heating effect, likely due to an influence of the microwaves on solution structuring. It is also shown that the process of microwave assisted slurry conversion crystallization for efficient manufacturing of co-crystals can be scaled up from 0.2 to at least 20 g with the same outcome. In the work, three new co-crystals, sulfamethazine–nicotinamide, sulfamerazine–anthranilic acid and sulfamerazine–salicylamide have been found. Their crystal structures were solved successfully using single crystal X-ray diffraction (SC-XRD). A further thorough characterization has been carried out by powder X-ray diffraction (PXRD), thermogravimetric analysis (TGA), differential scanning calorimetry (DSC) and Raman and FT-IR spectroscopy. It is shown that these co-crystals increase the equilibrium concentration of the drug compared to that of the respective pure solid forms. By Hirshfeld analysis and interaction energy calculations, the difference in stability, reactivity and rate of formation of co-crystals for sulfamethazine and sulfamerazine can be explained.


 Please wait while we load your content...
Please wait while we load your content...