Synthesis of polyheterocyclic 1,1-diboryltriazenes by γ-nitrogen insertion of azides into activated B–B single bonds†
Abstract
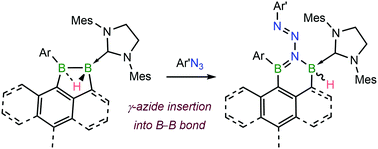
The γ-nitrogen insertion of arylazides into the B–B bond of electron-rich cyclic μ-hydridodiboranes stabilised by one N-heterocyclic carbene (NHC) ligand leads to the expansion of the central C3B2 ring, yielding unsymmetrical polyheterocyclic 1,1-diboryltriazenes. The 2-benzyl-bridged analogues undergo further NHC ring expansion and thermally induced loss of N2.


 Please wait while we load your content...
Please wait while we load your content...