A peptide–drug hydrogel to enhance the anti-cancer activity of chlorambucil†
Abstract
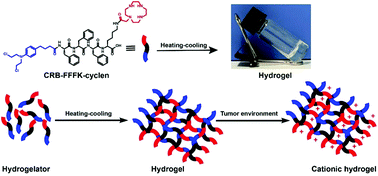
The clinical applications of nitrogen mustard antitumor drugs are limited by their poor aqueous solubility, poor cellular uptake, lack of targeting, and severe side effects. Cyclen could be protonated under physiological conditions, which may be beneficial for increasing cell membrane affinity and cellular uptake. Herein, a novel self-assembling peptide–drug conjugate was developed by conjugating chlorambucil (CRB) and cyclen to a self-assembling peptide. The resultant supramolecular hydrogel was prepared via a heating–cooling process and displayed improved aqueous solubility. Rheology, CD spectra, and transmission electron microscopy measurements indicated that the hydrogel with a β-sheet configuration and a nanofiber structure had favorable rheological properties. A cellular uptake experiment demonstrated that cyclen effectively increases the uptake of the resulting hydrogel by tumor cells. MTT results indicated that the hydrogel exhibited favorable inhibitory activities against A549, HeLa, and MCF-7 cancer cell lines and was less toxic towards 3T3 (normal cells). The results of γ-H2AX experiments showed that the obtained nanomedicine could induce significantly more DNA damage compared with free chlorambucil. Hematology analysis experiments revealed that the obtained nanomedicine has good biocompatibility. Our findings indicate that the self-delivery nanodrug system has clinical potential for cancer treatment.


 Please wait while we load your content...
Please wait while we load your content...