ZIF-67-derived Co3O4@carbon protected by oxygen-buffering CeO2 as an efficient catalyst for boosting oxygen reduction/evolution reactions†
Abstract
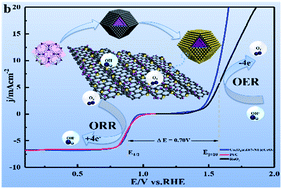
The synergies between transition metal oxides (bimetallic oxides) play important roles in determining the bifunctional catalytic activity for the oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) in alkaline media. This study uses a facile hydrothermal method for uniformly coating CeO2 shells on the surfaces of ZIF-67-derived porous Co3O4@Z67-NT (T-temperature, 500–900 °C) cores. Co3O4@Z67-N700@CeO2 exhibits excellent bifunctional (ORR/OER) catalytic activity with a ΔE [Ej=10(OER) − E1/2(ORR)] of 0.70 V. For ORR, Co3O4@Z67-N700@CeO2 exhibits a higher half-wave potential of 0.88 V (vs. RHE) than that of commercial Pt/C (0.87 V), which is attributed to the synergies between CeO2 (Ce3+) and Co3O4 (Co2+). The oxygen vacancies on CeO2 can enhance O2 adsorption on the interfaces to promote the activation of adsorbed O2 to O2−, and can alleviate O2 deficiency during ORR, thereby improving ORR activity. For OER, Co3O4@Z67-N700@CeO2 has a lower overpotential of 350 mV at 10 mA cm−2 and a higher Faraday efficiency of 92.2% than those of RuO2. An effective valence transition between Ce3+ and Ce4+ endows CeO2 with high electrical conductivity and oxygen storage capacity, which greatly promote charge transfer and the generation/smooth transport of highly active species (O22−/O−). Moreover, the interactions between CeO2 (Ce3+/Ce4+ and oxygen vacancies) and Co3O4 (Co3+/CoOOH) provide more electrochemically active sites for OER. This study provides a new strategy for constructing the stable core@shell structure based on MOF derivatives to improve the ORR/OER performance.


 Please wait while we load your content...
Please wait while we load your content...