Ni–Mo–O nanorod-derived composite catalysts for efficient alkaline water-to-hydrogen conversion via urea electrolysis†
Abstract
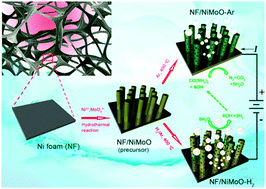
Photo/electrochemical splitting of water to hydrogen (H2) fuel is a sustainable way of meeting our energy demands at no environmental cost, but significant challenges remain: for example, the sluggish anodic reaction imposes a considerable overpotential requirement. By contrast, urea electrolysis offers the prospect of energy-saving H2 production together with urea-rich wastewater purification, whereas the lack of inexpensive and efficient urea oxidation reaction (UOR) catalysts places constraints on the development of this technique. Here we report a porous rod-like NiMoO4 with high oxidation states of the metal elements enabling highly efficient UOR electrocatalysis, which can be readily produced through annealing solid NiMoO4·xH2O as a starting precursor in Ar. This precursor gives the derived Ni/NiO/MoOx nanocomposite when switching the shielding gas from Ar to H2/Ar, exhibiting platinum-like activity for the hydrogen evolution reaction (HER) in alkaline electrolytes. Assembling an electrolytic cell using our developed UOR and HER catalysts as the anode and cathode can provide a current density of 10 milliamperes per square centimeter at a cell voltage of mere 1.38 volts, as well as remarkable operational stability, representing the best yet reported noble-metal-free urea electrolyser. Our results demonstrate the potential of nickel–molybdenum-based materials as efficient electrode catalysts for urea electrolysers that promises cost-effective and energy-saving H2 production.


 Please wait while we load your content...
Please wait while we load your content...