Electrochemical stability of non-aqueous electrolytes for sodium-ion batteries and their compatibility with Na0.7CoO2†
Abstract
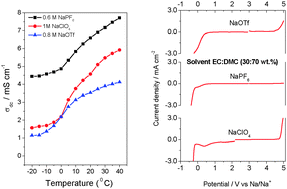
The present study compares the physico-chemical properties of non-aqueous liquid electrolytes based on NaPF6, NaClO4 and NaCF3SO3 salts in the binary mixture of ethylene carbonate (EC) and dimethyl carbonate (DMC). The ionic conductivity of the electrolytes is determined as a function of salt concentration and temperature. It is found that the electrolytes containing NaClO4 and NaPF6 exhibit ionic conductivities ranging from 5 mS cm−1 to 7 mS cm−1 at ambient temperature. The electrochemical stability window of the different electrolytes is studied by linear sweep voltammetry (LSV) and cyclic voltammetry (CV) measurements with respect to a variety of working electrodes (WE) such as glassy carbon (GC), graphite and a carbon gas diffusion layer (GDL). Electrolytes containing NaPF6 and NaClO4 are found to be electrochemically stable with respect to GC and GDL electrodes up to 4.5 V vs. Na/Na+, with some side reactions starting from around 3.0 V for the latter salt. The results further show that aluminium is preferred over different steels as a cathode current collector. Copper is stable up to a potential of 3.5 V vs. Na/Na+. In view of practical Na-ion battery systems, the electrolytes are electrochemically tested with Na0.7CoO2 as a positive electrode. It is inferred that the electrolyte NaPF6–EC : DMC is favorable for the formation of a stable surface film and the reversibility of the above cathode material.

 Please wait while we load your content...
Please wait while we load your content...