Relationship between molecular structures of uniquely designed C2-symmetric axially chiral dopants and their helical twisting properties in cholesteric liquid crystals†
Abstract
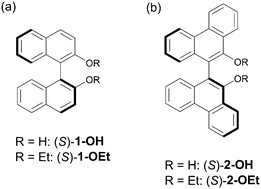
The induction of chirality in a liquid crystal (LC) can lead to the development of functional LC materials with enhanced properties. A chiral dopant is known to induce a molecular rearrangement and a subsequent helical twisting in the nematic LC host, affording a cholesteric liquid crystal (CLC). However, the chirality transfer mechanism has not been fully elucidated yet. In this study, we newly synthesized 9,9′-biphenanthrene-type chiral dopants ((S)-2s) following our work on binaphthyl-type chiral dopants with the aim of unveiling the chirality transfer mechanism. The molecular structures of the chiral dopants in the crystal were determined by XRD. Significantly unique Cotton effects were observed in (S)-2s, indicating that (S)-2s adopted a different conformation in solution from the binaphthyl-type chiral dopants. Interestingly, one of the (S)-2s dopants shows positive temperature dependence of helical twisting power (HTP) though most chiral dopants show negative temperature dependence. The effects of mixing chiral dopants having opposite temperature dependence of helical twisting power were also investigated. Finally, a CLC showing temperature independency was obtained by mixing two types of chiral dopants with a reverse trend of temperature dependence in HTP.
- This article is part of the themed collection: 2019 Journal of Materials Chemistry C HOT Papers


 Please wait while we load your content...
Please wait while we load your content...
