Improving selectivity of CO reduction via reducing the coordination of critical intermediates†
Abstract
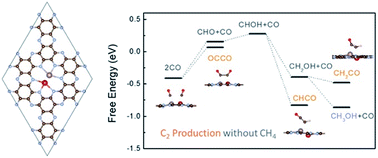
Electrochemical reduction of CO to value-added chemicals has attracted great interest. A challenge is to improve the selectivity towards C2 chemicals and suppress the formation of CH4 that is less valuable. Here using first-principles calculations, we show that CH4 formation can be suppressed via reducing the coordination of critical intermediates. This is demonstrated through the example of double metal atoms anchored on carbon nitride (C2N), which can efficiently catalyze the production of C2 chemicals, without CH4. The high selectivity originates from the weakened binding of the *CH/*CH2 intermediate with the metal atoms due to the reduced coordination, resulting in coupling with neighboring *CO and forming a C2 intermediate that can further transform into C2 products. Our work provides a new strategy to improve the selectivity of CO reduction and also suggests a promising candidate.
- This article is part of the themed collection: Journal of Materials Chemistry A Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...